Synthesis and Reactivity of ruthenium hexacationic prisms
(L. Paul, J. Furrer)
We have recently synthesized a number of water-soluble hexacationic metallaprisms that form hexanuclear cages. Notably, these metallaprisms were capable of encapsulating planar Pt and Pd acetylacetonate complexes. Interestingly, the metallaprisms exhibit a remarkable activity (IC50 = from 1 to 23 µM) against A2780 cancer cell lines, which increases with the encapsulation of planar Pt and Pd acetylacetonate complexes suggesting transport and leaching of the guest once inside the cell. Various experiments were subsequently undertaken to establish the nature of the species that are effectively transported into the cell, possible mechanisms of detoxification, as well as further information on the possible cellular target that may be related to their antitumor activity. The results showed that the prisms specifically interact with the thiol groups of Cys and GSH, and with the side chain of His, Lys, and Arg. The reactions with His, Lys, and Arg formed stable chelates. Our results suggest an inverse correlation between metallo-drug–protein interaction and cytotoxicity against tumour cells, since the most cytotoxic assembly reacts relatively slowly and incompletely with biomolecules, while the less cytotoxic assembly reacts rather quickly. Similarly to the mechanisms of action of numerous
Ru-drugs investigated so far, our results strongly suggest that these metallaprisms possess a different mechanism of cancer cell cytotoxicity as compared to Pt-drugs.
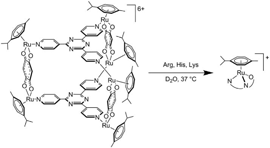
The metallaprism [(p-CH3C6H4Pri)6Ru6(tpt)2(dhbq)3]6+ disassembles upon addition of Arg, His, and Lys, thus forming stable (p-cymene)Ru amino acid derivatives. However, this metallaprism remains intact in the presence of Met, which is in contrast to other ruthenium-based drugs
... full size
Further Reading
- |
B. Therrien, G. Süss-Fink, P. Govindaswamy, |
- |
L. Paul, B. Therrien, J. Furrer |
- |
L. Paul, B. Therrien, J. Furrer J. Biol. Inorg. Chem. 17, 1053-1062, 2012. |
- |
F. Giannini, L. Paul, J. Furrer |
- |
L. Paul, B. Therrien, J. Furrer, J. Organomet |